History and Chemistry of Dryer Sheets
Ever wondered why we toss dryer sheets along with wet laundry in the dryer? Or how do these thin sheets soften and freshen our clothes?
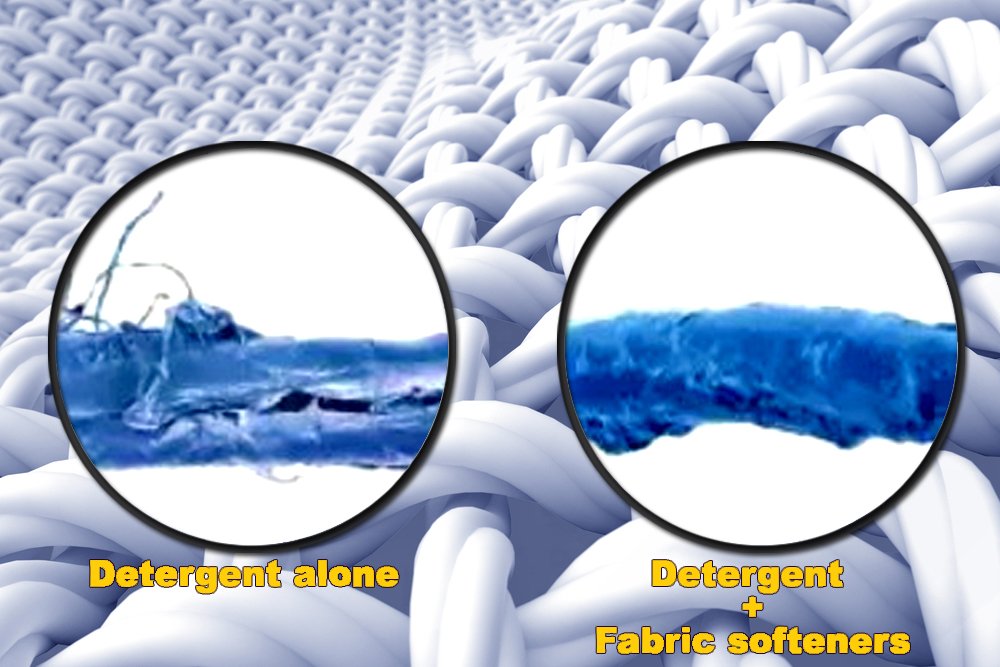
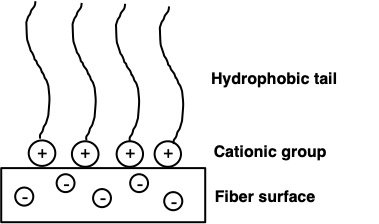
Dryer sheets belong to a special class of materials called fabric softeners. Some synthetic detergents strip off natural oils and waxes from fabric fibers, which make clothes harsh and stiff. But in the 1930's, dryer sheets were invented to counteract the effects of synthetic detergents!.[1] Fabric softeners contain positively charged (cationic) groups. These cationic groups attach to the negatively charged surface of the fibers in your clothes by electrostatic attraction (which is like a force that pulls unlike charges together) and neutralize the negative charge on the surface. The long chain molecules in the softener then orient towards the outside of the fiber to create a barrier, making fibers smooth, flexible and moisture-retaining. In this way fabric softeners also prevent the buildup of static charges on clothes which can make them uncomfortable to wear. It is these static charges that cause clothes to stubbornly cling to each other post laundry.
How were dryer sheets invented? [2]
Since the liquid fabric softeners are positively charged, they must be added during the rinse cycle, or they can react with negatively charged surfactants in the detergent and form salts by precipitation when added to the wash cycle. This problem baffled Conrad J Gaiser, a chemist experienced with soap and detergent chemistry. To save his wife the effort of going down the stairs in his multistory house to catch the final rinse cycle to add fabric softener, he created dryer sheets by treating small pieces of cotton flannel with fabric softener. When put in the dryer along with wet laundry the heat and moisture warmed up the softener and allowed it to spread on clothes. In 1969, Gaiser received a US Patent for his invention of dryer sheets, and he called his prototype Tumble Puffs. [3, 4] He then sold the rights to Procter and Gamble (P&G) who perfected the product and launched it in 1975. [2]
What is the chemistry behind dryer sheets?
Schematic representation of a cationic softener acting on a negatively charged fiber surface
A representative quaternary ammonium salt chemistry
Today, many brands manufacture dryer sheets using different formulas to achieve similar goals of softening clothes, preventing static buildup, and making clothes smell good. The base of most dryer sheets is a non-woven polyester material coated with a softening agent which has a long hydrophobic (water-repelling) tail. Common softening agents include fatty acids, fatty alcohols and alcohol ethoxylates. [2] It is also important that the dryer sheets do not soften in the box, so the softening agent must have has a high melting point. P&G uses quaternary ammonium salts (a representative chemistry in shown in the picture on the side) as softening agents in its dryer sheets whereas Unilever uses stearic acid as softening agent in its dryer sheets. [2] You can read the back of the dryer sheet box to see what softening agent chemistry your brand of dryer sheet uses.
So, is it just the base and softener chemistry that determines the performance of a dryer sheet? Not quite, another prerequisite is that the softening agent must spread evenly on clothes in the dryer and for this, some inorganic clay compounds are added to control the spreading (defined by a property called viscosity) of fabric softener as it melts in the dryer to allow it to coat the fabric uniformly. [2] Similarly, fragrance molecules, particularly those that can survive high temperatures in dryers are used to impart fragrance to clothes. These molecules can also be enclosed within other molecules for a controlled release to minimize loss in the dryer due to evaporation.
Are dryer sheets suitable for all laundry?
The answer is no. Dryer sheets and fabric softeners will reduce the absorbing capacity of towels. [5] They can also damage microfibers commonly used in towels. Similarly, dryer sheets will reduce the moisture absorbing capacity of athletic sportswear, nylon, spandex etc. The waxy coating from the dryer sheets will also reduce the flame retardant capacity of clothing and hence it should not be used on children's clothing. Other than laundry, dryer sheets can be used [3] as insect repellents, to remove musty smell from books, as air fresheners, for scrubbing shower walls to remove soap scum and for removing pet hair from furniture.
Other interesting links to learn more about fabric softeners:
https://www.scienceabc.com/eyeopeners/how-do-fabric-softeners-work.htm
References:
Chiweshe, A.; Crews, P. C., Influence of household fabric softeners and laundry enzymes on pilling and breaking strength. Textile Chemist and Colorist and American Dyestuff Reporter 2000, 32, 41-47.
What's that stuff? Dryer Sheets. https://cen.acs.org/articles/86/i15/Dryer-Sheets.html (accessed 11/16/2021).
Tumble Puffs? http://patentplaques-blog.com/first-dryer-sheet-patent-tumble-puffs/ (accessed 11/16/2021).
Dryer Sheets — Is There An Alternative? https://www.theday.com/article/20120227/usr04/120229717 (accessed 11/16/2021).
Fabric Softeners And Dryer Sheets: Myths vs Facts. https://household-tips.thefuntimesguide.com/fabric-softener/ (accessed 11/16/2021).
Image credit for article icon: https://cen.acs.org/articles/86/i15/Dryer-Sheets.html